The Case for WHO PQ in Nigeria
The attainment of WHO PQ by the pharmaceutical manufacturers in Nigeria will establish their capability to produce medicines that consistently meet stringent standards of quality in line with WHO specifications and global standards.
This will provide opportunities for increased sales and market access by conferring eligibility for international, donor-sponsored tenders for medicines; improved capacity to manufacture products for entry into stringently regulated markets; increased potential to compete successfully for contract manufacture for local markets; and faster registration. Thus, facilitating international procurement and distribution of locally produced medicines in Nigeria.
Annually billions of dollars worth of products are donated to Africa without local participation in the tender because they are not WHO prequalified.
QUESTIONNAIRE FOR PHARMACEUTICAL MANUFACTURERS
This questionnaire is intended to facilitate the process of evaluating pharmaceutical manufacturers interested in participating in the above-mentioned program. The intention is to identify manufacturers capable of manufacturing antimalarial medicines that are compliant with World Health Organization (WHO) Good Manufacturing Practices (GMP), and successful prequalification of the medicinal product.
Excepts from our stakeholders
Stake Holders

Dr. Osagie Ehanire
Honourable Minister of Health
I am pleased to see this initiative taking place in Nigeria’s health sector. It shows that production, regulation and supply of quality assured medicines is a shared endeavour of the public and private sectors. The concerted effort of both stakeholders is essential to assuring Universal Health Coverage for better population health and well-being, as well as addressing needs for protection of citizens from public health challenges

Hon. Ekumankama Joseph Nkama
Minister of State for Health
By attaining WHO PQ, pharmaceutical manufacturers in Nigeria will establish their capability to produce medicines that consistently meet stringent standards of quality in line with global standards. Thus, facilitating international procurement and distribution of locally produced medicines in Nigeria

Alhaji. Mahmuda Mamman
Permanent Secretary Federal Ministry of Health
This is a golden opportunity that Nigerian pharmaceutical manufacturers must leverage on to increase their capacity for local manufacturing, thereby strengthening Nigeria’s health system for better epidemic preparedness and response

Dr. Perpetual Uhomoibhi
National Coordinator, National Malaria Elimination Programme (NMEP)
The National Malaria Elimination Programme is keen to ensure more local manufacturers of antimalarial commodities attain WHO Pre-qualification and Good Manufacturing Practice certification and will work with the Bloom Public Health and NIPRD to achieve this in line with Global best practices

Dr. Obi Adigwe
NIPRD Director General
After close to a decade in planning, our initiative to catalyze Nigeria’s emergence as a world leader in Pharma manufacturing has finally begun. The capacity we will build in the Industry is key to achieving Medicines’ Security. Ultimately, this will not only improve Health & Socioeconomic indices, it will also underpin Nigeria’s emergence as the Pharma Hub within the AfCFTA.

Prof. Chimezie Anyakora
CEO Bloom Public Health
I am really excited to be part of this huge landmark in Nigeria’s journey towards being a global player in the pharmaceutical sector. This is certainly the biggest intervention so far. Its uniqueness is that it is internally driven. It shows a great leadership by the country
Challenges confronting WHO PQ in Nigeria
One of the major barriers to achieving WHO PQ in Nigeria is the high upfront cost. Pharmaceutical manufacturers would need to invest several millions of dollars to upgrade their factories and to hire or train qualified professional and technical personnel in order to meet WHO PQ requirements. Unfortunately, without the support from government or donor agencies, most Nigerian pharmaceutical companies lack the capacity for such enormous capital investment. Another significant barrier is the poor technical support and limited expertise about achieving WHO PQ. Most Nigerian pharmaceutical companies have limited information on the specific requirements for WHO PQ and the application process. Attaining WHO PQ is a highly technical process, requiring comprehensive technical and professional support from experts in the field.
Proposed solution - Transformational change for the pharmaceutical industry in Nigeria
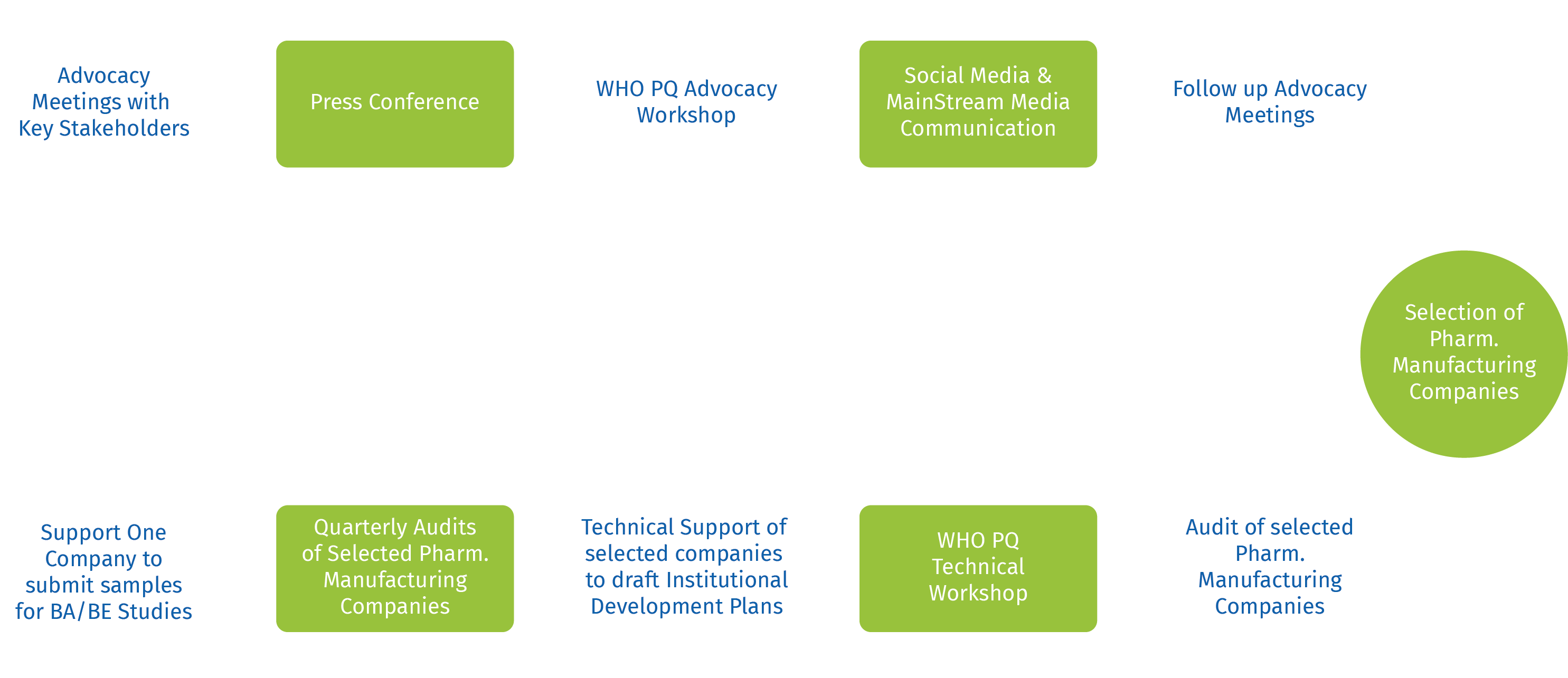
This project is by far Nigeria’s biggest pharmaceutical intervention and will achieve the first ever WHO PQ of pharmaceutical companies in Nigeria. It will have a tremendous impact on the Nigerian health system and build a national capacity for sustainable manufacturing, as well as position the pharmaceutical industry in Nigeria as a global competitor. Work plan starting from - Selection
WHO PQ Workplan
The Overview
WHO PQ
Benefit & Impact
WHO PQ will create huge public health and economic impact in Nigeria by ensuring an increased availability of quality, efficacious and safe medicines and reduce over reliance on imports for public health interventions. Therefore, this ongoing initiative by NIPRD and Bloom Public Health is a golden opportunity that Nigerian pharmaceutical companies must leverage to increase their capacity for local manufacturing and strengthen Nigeria’s pharmaceutical sector.